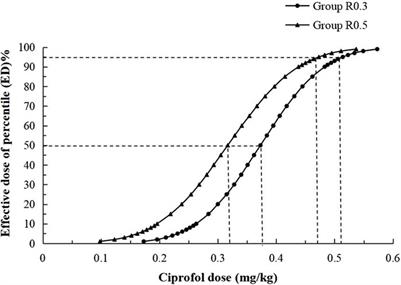
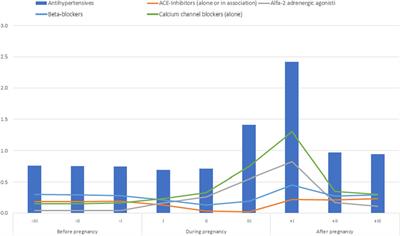
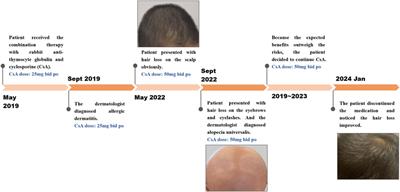
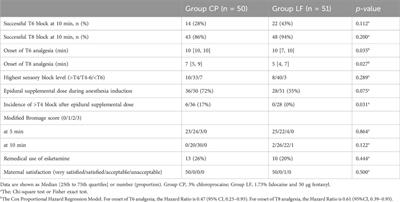
Clinical Trial
Published on 16 Oct 2024
Pharmacokinetics and bioequivalence of two formulations of mifepristone tablets in healthy Chinese subjects under fasting conditions: a single-center, open, randomized, single-dose, double-period, two-sequence, crossover trial
in Obstetric and Pediatric Pharmacology
- 1,060 views