Clinical Trial
Published on 17 May 2024
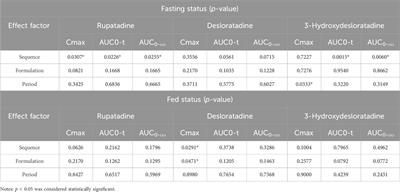
A single-dose, randomized, open-label, four-period, crossover equivalence trial comparing the clinical similarity of the proposed biosimilar rupatadine fumarate to reference Wystamm® in healthy Chinese subjects
in Drug Metabolism and Transport
- 1,540 views
- 3 citations