Study Protocol
Published on 29 Jul 2022
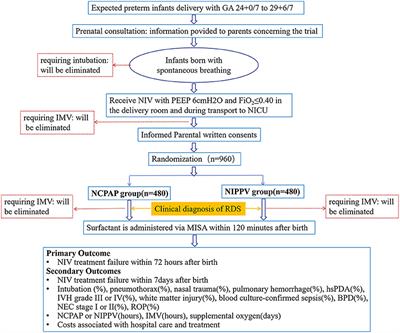
A multicenter, randomized controlled, non-inferiority trial, comparing nasal continuous positive airway pressure with nasal intermittent positive pressure ventilation as primary support before minimally invasive surfactant administration for preterm infants with respiratory distress syndrome (the NIV-MISA-RDS trial): Study protocol
in Pediatric Pulmonology
- 2,489 views
- 2 citations