Perspective
Published on 01 Nov 2023
Fecal microbiota transplantation—could stool donors’ and receptors’ diet be the key to future success?
in Gastrointestinal Infection
- 1,351 views
- 1 citation
Perspective
Published on 01 Nov 2023
in Gastrointestinal Infection

Editorial
Published on 24 Oct 2023
in Gastrointestinal Infection
Brief Research Report
Published on 24 Oct 2023
in Gastroenterology and Cancer
Original Research
Published on 23 Oct 2023
in Therapy in Gastroenterology
Original Research
Published on 18 Oct 2023
in Therapy in Gastroenterology
Review
Published on 16 Oct 2023
in Gastrointestinal Infection
Case Report
Published on 11 Oct 2023
in Endoscopy
Systematic Review
Published on 10 Oct 2023
in Gastroenterology and Cancer
Review
Published on 03 Oct 2023
in Therapy in Gastroenterology
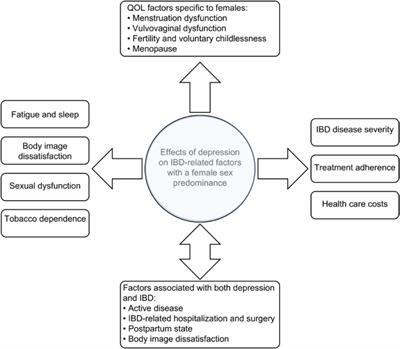
Review
Published on 03 Oct 2023
in Endoscopy

Mini Review
Published on 19 Sep 2023
in Gastroenterology and Cancer
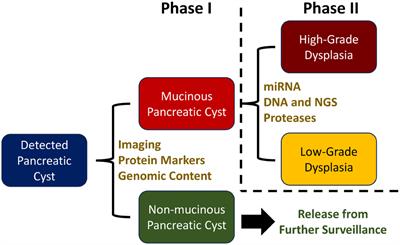
Original Research
Published on 14 Sep 2023
in Gastrointestinal Infection
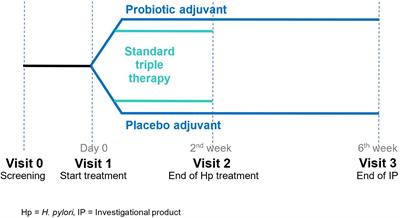
Original Research
Published on 11 Sep 2023
in Endoscopy

Original Research
Published on 05 Sep 2023
in Endoscopy
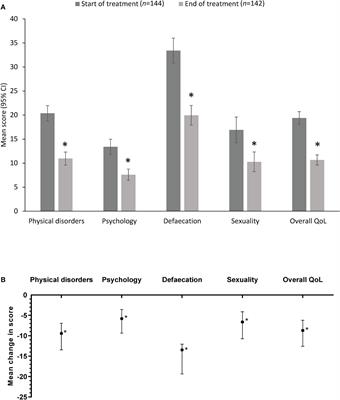
Editorial
Published on 21 Aug 2023
in Endoscopy
Case Report
Published on 21 Aug 2023
in Gastroenterology and Cancer